Identify the statement below that is true about chemical reactions – Delving into the realm of chemical reactions, this discourse embarks on an illuminating journey to decipher their intricate nature and profound implications. Chemical reactions, the cornerstone of chemistry, are captivating processes that involve the transformation of substances into new entities, accompanied by energy changes and the rearrangement of atoms and molecules.
This exploration unveils the fundamental principles governing these reactions, their diverse applications, and their profound impact on our world.
Chemical reactions encompass a vast array of phenomena, from the combustion of fuels to the synthesis of life-saving drugs. They play a pivotal role in shaping our physical environment, driving industrial processes, and sustaining biological systems. Understanding the intricacies of chemical reactions empowers us to harness their potential for the advancement of science and technology, while also fostering a deeper appreciation for the intricate tapestry of the natural world.
Chemical Reactions: Types and Characteristics

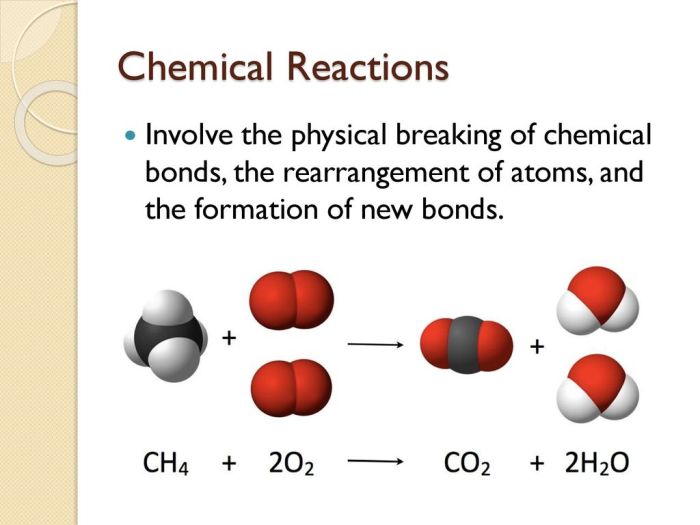
Chemical reactions involve the transformation of substances into new substances with different chemical compositions. These reactions are characterized by the breaking and formation of chemical bonds, leading to the rearrangement of atoms and molecules.
Types of Chemical Reactions, Identify the statement below that is true about chemical reactions
- Combination reactions:Two or more substances combine to form a single product (e.g., 2H 2+ O 2→ 2H 2O).
- Decomposition reactions:A single compound breaks down into two or more simpler substances (e.g., 2HgO → 2Hg + O 2).
- Single-replacement reactions:One element replaces another element in a compound (e.g., Zn + 2HCl → ZnCl 2+ H 2).
- Double-replacement reactions:Ions from two different compounds exchange places, forming two new compounds (e.g., AgNO 3+ NaCl → AgCl + NaNO 3).
Distinguishing Chemical Reactions from Physical Changes
Chemical reactions involve a change in the chemical composition of substances, while physical changes involve only changes in their physical properties (e.g., shape, size, color). Chemical reactions are irreversible, while physical changes are reversible.
Popular Questions: Identify The Statement Below That Is True About Chemical Reactions
What are the key characteristics of chemical reactions?
Chemical reactions involve the rearrangement of atoms and molecules, resulting in the formation of new substances with distinct properties. They are typically accompanied by energy changes, either in the form of heat release (exothermic) or heat absorption (endothermic).
How do chemical reactions differ from physical changes?
Chemical reactions involve the breaking and forming of chemical bonds, leading to the creation of new substances with different chemical compositions. Physical changes, on the other hand, involve changes in the physical state of a substance, such as melting, freezing, or boiling, without altering its chemical composition.
What factors influence the rate of chemical reactions?
The rate of chemical reactions is influenced by various factors, including the concentration of reactants, temperature, surface area, and the presence of catalysts. Increasing the concentration of reactants, raising the temperature, increasing the surface area, and adding catalysts can all accelerate the reaction rate.