Development of atomic theory worksheet – Embark on an enthralling journey into the realm of atomic theory with our comprehensive worksheet. This meticulously crafted resource unravels the intricate tapestry of atomic structure, bonding, and nuclear chemistry, inviting you to delve into the fundamental building blocks of our universe.
Prepare to unravel the groundbreaking discoveries that shaped our understanding of matter, from the ancient Greeks to the modern era. Our worksheet will guide you through the contributions of key scientists, tracing the evolution of atomic theory over time.
Development of Atomic Theory Timeline
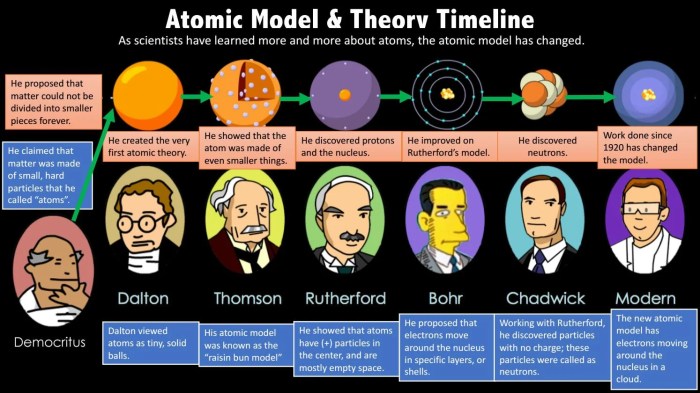
The development of atomic theory has been a gradual process, with many key milestones along the way.
- Scientist:Democritus
- Year:400 B.C.
- Discovery:Proposed that all matter is made up of tiny, indivisible particles called atoms.
- Impact:Introduced the concept of atoms, but his ideas were not widely accepted.
- Scientist:John Dalton
- Year:1803
- Discovery:Developed the atomic theory, which stated that all matter is composed of atoms, atoms are indivisible and indestructible, and all atoms of a given element are identical in mass and other properties.
- Impact:Established the foundation of modern atomic theory.
- Scientist:J.J. Thomson
- Year:1897
- Discovery:Discovered the electron, a negatively charged particle within atoms.
- Impact:Led to the discovery of subatomic particles and the “plum pudding” model of the atom.
- Scientist:Ernest Rutherford
- Year:1911
- Discovery:Conducted the gold foil experiment, which led to the discovery of the atomic nucleus.
- Impact:Revolutionized the understanding of atomic structure and led to the development of the nuclear model of the atom.
- Scientist:Niels Bohr
- Year:1913
- Discovery:Proposed the Bohr model of the atom, which described electrons orbiting the nucleus in specific energy levels.
- Impact:Introduced the concept of energy levels and quantum mechanics in atomic theory.
- Scientist:Erwin Schrödinger
- Year:1926
- Discovery:Developed the quantum mechanical model of the atom, which uses wave functions to describe the behavior of electrons.
- Impact:Provided a more accurate and sophisticated understanding of atomic structure.
FAQ Explained: Development Of Atomic Theory Worksheet
What are the key milestones in the development of atomic theory?
Our worksheet Artikels the major milestones, including Dalton’s atomic theory, Thomson’s discovery of electrons, Rutherford’s nuclear model, and Bohr’s atomic model.
How does atomic structure impact the properties of elements?
The worksheet explains how the number of protons, neutrons, and electrons determines an element’s atomic number, mass number, and isotopic variations, influencing its chemical and physical properties.
What are the different types of chemical bonds?
Our worksheet covers ionic, covalent, and metallic bonds, detailing their formation, properties, and the compounds they form.