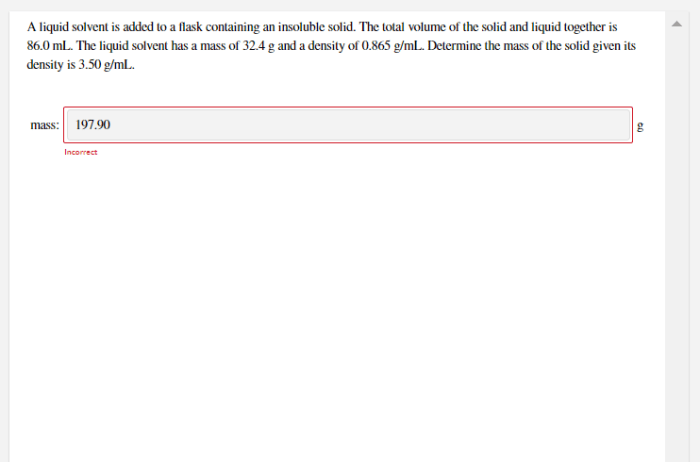
When a liquid solvent is added to a flask, a captivating dance of physical and chemical changes unfolds. This process, widely employed in various scientific and industrial domains, offers a fascinating glimpse into the intricate interplay between solvents and solutes.
As the solvent is introduced, the flask’s contents undergo a series of observable transformations, ranging from subtle shifts in color to more pronounced changes in texture and viscosity. These alterations stem from the solvent’s unique properties, such as polarity and boiling point, which influence its interactions with the dissolved substance.
Physical Changes

When a liquid solvent is added to a flask, several physical changes may be observed. These changes can include alterations in color, texture, and viscosity.
Color changescan occur when the solvent interacts with the solute. For example, adding water to a solution of potassium permanganate will cause the solution to turn from purple to pink. This color change is due to the formation of a new compound, potassium permanganate hydrate.
Texture changescan also occur when a solvent is added to a flask. For example, adding water to a solution of sugar will cause the solution to become thicker and more viscous. This change in texture is due to the formation of hydrogen bonds between the water molecules and the sugar molecules.
Viscosity changescan also occur when a solvent is added to a flask. Viscosity is a measure of the resistance of a fluid to flow. Adding a solvent to a solution will decrease the viscosity of the solution. This is because the solvent molecules act as a lubricant, making it easier for the solution to flow.
Common Liquid Solvents
- Water is a common liquid solvent that is used to dissolve a wide variety of substances.
- Alcohol is another common liquid solvent that is used to dissolve organic compounds.
- Chloroform is a liquid solvent that is used to dissolve nonpolar compounds.
Chemical Reactions: A Liquid Solvent Is Added To A Flask
The addition of a liquid solvent to a flask can potentially initiate various chemical reactions, depending on the nature of the solvent and the solute. The likelihood of a reaction occurring is influenced by several factors, including the polarity of the solvent, the solubility of the solute, and the presence of catalysts or inhibitors.
One common type of chemical reaction that can occur is dissolution, where the solute dissolves into the solvent, forming a homogeneous mixture. This process is typically driven by the solvation of the solute molecules by the solvent molecules, which involves the formation of intermolecular interactions such as hydrogen bonding, dipole-dipole interactions, or van der Waals forces.
Factors Influencing Chemical Reactions
The polarity of the solvent is a crucial factor in determining the likelihood of a reaction. Polar solvents, such as water or methanol, have a net electrical charge separation, which allows them to dissolve ionic compounds and polar molecules. Nonpolar solvents, such as hexane or chloroform, have no net charge separation and are more effective at dissolving nonpolar molecules.
The solubility of the solute in the solvent is another important factor. A solute is considered soluble if it can dissolve in a solvent to form a homogeneous mixture. The solubility of a solute is influenced by its molecular structure, polarity, and intermolecular interactions.
Generally, polar solutes are more soluble in polar solvents, while nonpolar solutes are more soluble in nonpolar solvents.
Specific Examples
Specific examples of chemical reactions that can be initiated by the addition of a liquid solvent include:
- Acid-base reactions:The addition of an acidic solvent, such as hydrochloric acid, to a solution of a base, such as sodium hydroxide, can result in the formation of a salt and water.
- Precipitation reactions:The addition of a solvent that decreases the solubility of a solute can cause the solute to precipitate out of solution.
For example, the addition of ethanol to a solution of sodium chloride can cause the sodium chloride to precipitate out of solution.
- Redox reactions:The addition of a solvent that can act as an oxidizing or reducing agent can initiate redox reactions.
For example, the addition of potassium permanganate to a solution of ferrous sulfate can result in the oxidation of ferrous ions to ferric ions.
Solvent Properties
The choice of liquid solvent for a particular application depends on its properties. These properties include polarity, boiling point, and solubility.
Polarity is a measure of the uneven distribution of electrical charge within a molecule. Polar solvents have a dipole moment, which is a vector that points from the positive end of the molecule to the negative end. Nonpolar solvents have no dipole moment.
Boiling point is the temperature at which a liquid boils. The boiling point of a solvent is determined by its intermolecular forces. Solvents with strong intermolecular forces have high boiling points, while solvents with weak intermolecular forces have low boiling points.
Solubility is the ability of a solute to dissolve in a solvent. The solubility of a solute in a solvent is determined by the polarity of the solute and the solvent. Polar solutes dissolve in polar solvents, and nonpolar solutes dissolve in nonpolar solvents.
Table of Solvent Properties
| Solvent | Polarity | Boiling Point (°C) | Solubility (g/100 mL) |
|---|---|---|---|
| Water | Polar | 100 | 26.5 |
| Ethanol | Polar | 78.3 | 12.5 |
| Hexane | Nonpolar | 68.7 | 0.001 |
| Benzene | Nonpolar | 80.1 | 0.007 |
Applications

Liquid solvents find extensive applications across various industries, owing to their unique ability to dissolve or disperse other substances. They play a crucial role in chemical reactions, manufacturing processes, and medical applications.
Chemistry
- Extraction:Solvents are used to extract specific compounds from mixtures or natural sources. For instance, organic solvents are employed in the extraction of essential oils from plants.
- Purification:Solvents are utilized to purify substances by selectively dissolving impurities and allowing them to be removed. Recrystallization is a common purification technique that involves dissolving the impure substance in a suitable solvent and then recrystallizing it to obtain a purer form.
- Chromatography:Solvents are the mobile phase in chromatography techniques, which are used to separate and analyze mixtures. Different solvents have different polarities, allowing for the separation of compounds based on their affinity for the solvent.
Manufacturing
- Paints and Coatings:Solvents are used as carriers for pigments and resins in paints and coatings. They help to dissolve and disperse the components, ensuring a smooth and uniform application.
- Adhesives:Solvents are often added to adhesives to adjust their viscosity and drying time. They can also help to improve the adhesion between surfaces.
- Cleaning:Solvents are widely used for cleaning and degreasing purposes. They can dissolve and remove dirt, grease, and other contaminants from surfaces.
Medicine, A liquid solvent is added to a flask
- Drug Delivery:Solvents are used as carriers for drugs in various pharmaceutical formulations. They can help to dissolve drugs and improve their absorption and bioavailability.
- Disinfectants:Some solvents, such as alcohol and hydrogen peroxide, have antiseptic and disinfectant properties. They are used to kill microorganisms on surfaces and in medical instruments.
- Anesthetics:Certain solvents, such as ether and chloroform, were historically used as anesthetics during surgical procedures.
Safety Considerations

Liquid solvents pose potential hazards due to their flammability, toxicity, and volatility. Proper handling, storage, and disposal are crucial to minimize risks.
Ventilation is essential to prevent solvent vapors from accumulating and creating an explosive atmosphere. Use fume hoods or well-ventilated areas for handling solvents. Protective gear, including gloves, eye protection, and respirators, should be worn to prevent skin contact, inhalation, and eye irritation.
Waste Disposal
Solvents must be disposed of properly to avoid environmental contamination. Contact a licensed waste disposal company for guidance on specific disposal methods. Never pour solvents down the drain or dispose of them in landfills.
Safe Handling and Storage
Store solvents in tightly sealed containers in a cool, well-ventilated area away from heat sources and incompatible materials. Keep solvents away from open flames and sparks. Ground all equipment to prevent static discharge.
Detailed FAQs
What factors influence the likelihood of a chemical reaction when a liquid solvent is added?
Factors such as solvent polarity, solute solubility, and temperature play a significant role in determining the probability of a chemical reaction.
How do solvents contribute to various industrial processes?
Solvents serve as essential components in industries such as chemistry, manufacturing, and medicine, facilitating reactions, extraction processes, and the production of a wide range of products.
What safety precautions should be taken when handling liquid solvents?
Proper ventilation, protective gear, and adherence to waste disposal guidelines are crucial for minimizing the potential hazards associated with liquid solvents.